Current Projects
 LOHC Powertrains to Decarbonize Long-Haul Trucks
LOHC Powertrains to Decarbonize Long-Haul Trucks
In this project, we propose a concept design featuring on-board dehydrogenation of liquid organic hydrogen carriers to offer a practical, cost competitive alternative to diesel powertrains in an effort to decarbonize long-haul trucking. The project scope includes powertrain modelling and technical design, technoeconomic assessment, and prototype buildout for an experimental demonstration.
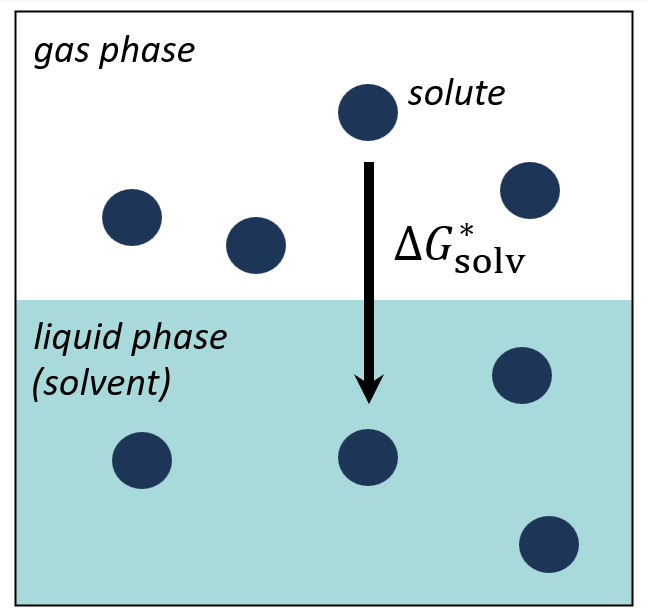 Solvation and Related Quantities
Solvation and Related Quantities
This project aims to develop methods for predicting thermodynamic and kinetic properties of liquid-phase systems. Our ongoing work involves dataset generation, deep learning, and quantum chemistry, encompassing properties such as solubility, rate constants, acid dissociation constants, and solvation free energies.
 Decarbonization of Ethylene Production
Decarbonization of Ethylene Production
In this project, we develop new processes for sustainable ethylene production. We identify promising replacement technologies for steam cracking, guided by techno-economic analysis and life-cycle assessment of our newly developed processes and existing alternatives. Furthermore, we develop schemes to retrofit and repurpose existing steam crackers with the goal of reducing GHG emissions to direct the industry in the transition to a more sustainable ethylene production.
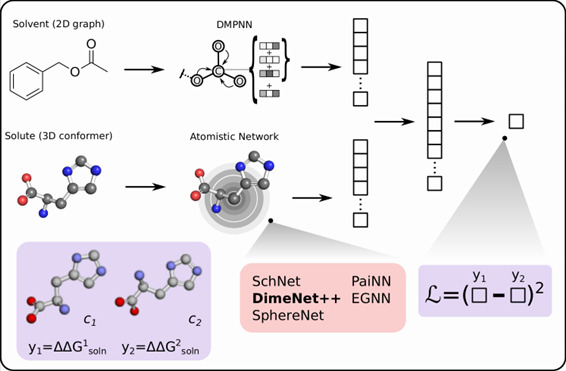 Accelerated Prediction of Conformer Solution Free Energies with Machine Learning
Accelerated Prediction of Conformer Solution Free Energies with Machine Learning
In this project, machine learning is used to predict the relative solution free energies of solute conformers in different solvents, enabling rapid identification of the main thermodynamically relevant conformers.
 Exploring Ammonia as a Fuel for Emissions Reduction
Exploring Ammonia as a Fuel for Emissions Reduction
This project aims to assess the feasibility of ammonia as a fuel for reduction of emissions. There are two components, one using first-principles kinetics and the Reaction Mechanism Generator to construct a detailed mechanism for ammonia combustion, and another using techno-economic analysis (TEA) and life-cycle analysis (LCA) to assess the feasibility of ammonia relative to alternative low carbon processes like hydrogen or carbon-capture.
 Predicting Active Pharmaceutical Ingredients (API) Degradation
Predicting Active Pharmaceutical Ingredients (API) Degradation
This project aims to automatically predict the chemical stability of an API. Specifically, we are interested in predicting, with quantitative accuracy, the degradation products, pathways, and rates of API oxidation in liquid phase at or close to room temperature.
 Automatic Chemical Reaction Discovery
Automatic Chemical Reaction Discovery
This project is developing robust and efficient computational methods to automatically and systematically search for new and important chemical reactions.
 Predictive Kinetics for Gas and Liquid Systems
Predictive Kinetics for Gas and Liquid Systems
For most technologically important systems, including combustion, pyrolysis, and atmospheric oxidation of organic compounds, it is very difficult to construct a reliable kinetic model. There are typically hundreds of reaction intermediates, and only a small fraction of the rate parameters are known experimentally. However, advances in computational chemistry, numerical methods, and computer hardware have now made it possible to construct and solve kinetic models which can reliably modeling these complicated systems. For more information, download the slides from Prof. Green’s keynote talk at the 8th International Conference on Chemical Kinetics.
In the Chemical Dynamics Laboratory (CDL) at MIT, we have designed and built a novel apparatus to measure the temperature- and pressure-dependent chemical kinetics and the product-branching ratios of gas-phase radical reactions that are important in combustion.
Past Projects
 Exascale Computing Project
Exascale Computing Project
The goal of this project is to develop an automatic reaction mechanism generation scheme using exascale computations to construct high fidelity chemical models for the simulation of RCCI combustion including multi-component fuel chemistry at low-temperature, high pressure, and sooting flames.

This project is on developing and refining chemical kinetic models for candidate biofuels and improving model construction and refinement processes.
 Computer Assisted Organic Synthesis Planning
Computer Assisted Organic Synthesis Planning
This project aims to automatically predict organic synthesis routes for arbitrary specified molecules from feed-stocks and execute these syntheses with minimal human intervention.
 Fundamentals of Polymer Fouling in Ethylene Crackers
Fundamentals of Polymer Fouling in Ethylene Crackers
Polymer fouling is a ubiquitous problem in ethylene cracking plants. This projects aims to elucidate the fundamentals of polymer fouling and expedite the development of efficacious mitigation strategies. Both tasks will be guided by Reaction Mechanism Generator (RMG).
 Naphthalene and Soot Emissions in Jet Engines
Naphthalene and Soot Emissions in Jet Engines
Particulate emissions from aviation are a growing area of interest, particularly with regards to the potential contribution to climate change via radiative forcing and the health risks associated with high levels of particular matter. We are interested in determining the degree to which naphthalene contributes to soot emissions in jet engines.
 Mobility of the Future – Vehicles & Fuels
Mobility of the Future – Vehicles & Fuels
The MIT Mobility of the Future project is a multidisciplinary effort to project how global ground transportation will work in 2050. It is funded by an industrial consortium, and Prof. Green is the Faculty Chair of the effort. Our group is in charge of the technoeconomic parameters around Fuels and Vehicles, and we are collaborating closely with the EPPA team. Other participants in the project are focused on consumer preferences, transportation mode options (particularly in urban areas), and the system dynamics that control the timescale of transitions in the transportation system (e.g. from combustion vehicles to battery-powered vehicles).
 Reaction Mechanisms of Heteratomic Species
Reaction Mechanisms of Heteratomic Species
The open-source software Reaction Mechanism Generator (RMG), developed by our group in Python and Java, explores networks of reaction mechanisms and discovers the subset of species and reactions that are able to represent the macroscopic properties (e.g. ignition delay) of the complete reaction network. We are extending RMG to account for heteroatoms, such as sulfur and nitrogen, to extend RMG’s range of chemistry.
 Hydrogen Sulfide Decomposition
Hydrogen Sulfide Decomposition
We are developing new processes to recover hydrogen gas from hydrogen sulfide. Hydrogen sulfide is produced on a large scale during the hydrodesulfurization of hydrocarbons.
 High-Fidelity Model for Endothermic Jet Fuel Cracking and Coking
High-Fidelity Model for Endothermic Jet Fuel Cracking and Coking
The goal of this research is to develop a computer simulation of the chemical and physical phenomena occurring in the cooling channels, with a focus on the cracking reactions which dominate the heat absorption and the reactions which could lead to coke formation.

Natural gas is an increasingly plentiful and economical fuel. However, widespread adoption in multiple applications requires a strong fundamental understanding of its combustion. While models that describe natural gas combustion at atmospheric conditions exist, we aim to build on these models to accurately simulate combustion at the more extreme conditions present in an internal combustion engine. In our study, Extinction Strain Rate (ESR) prediction is used for validation and mechanism development.
We are interested in understanding the fundamental mechanisms for upgrading Arabian Heavy Crude using supercritical water and developing a model to measure, predict, and quantify the upgrading process.
 Kinetics and Mechanisms of Heterogeneous Catalysis
Kinetics and Mechanisms of Heterogeneous Catalysis
Catalysts are crucial to transform biomass derived bio-oils into intermediates for liquid transportation fuels. We are studying the reaction kinetics and mechanisms during hydro-deoxygenation of such oxygenates derived from biomass using large scale Density Functional Theory (DFT) calculations. The discovered kinetics and mechanisms allow us to generate leads for experimental catalysis studies and to computationally design new catalyst materials.
 Fungi Generated Ketone Biofuels
Fungi Generated Ketone Biofuels
Several strains of endophytic fungi have been shown to directly convert lignocellulosic biomass to a wide range of compounds, including ketones, ethers, and terpenoids, that could serve as potential biofuels. Understanding the combustion chemistry of these compounds is key to providing fuel target recommendations for synthetic biologists to optimize and scale-up these fungi metabolic pathways.
 Sulfur Chemistry in Carbon Neutral Combustion
Sulfur Chemistry in Carbon Neutral Combustion
Oxyfuel combustion may facilitate carbon capture techniques, leading to a possible carbon-neutral combustion; however, it also affects sulfur chemistry during combustion of sulfur-containing fuels. Sulfur oxides (SOx) have adverse effects on human health and the environment. We aim to understand SOx formation in oxyfuel combustion using computational tools.
 Combustion Modeling of Jet Fuel: JP-10
Combustion Modeling of Jet Fuel: JP-10
JP-10 (exo-tetrahydrodicyclopentadiene) is an important fuel in military applications, particularly in the development of air-breathing propulsion systems. One the main advantages of JP-10 as a fuel is its high volumetric energy density; it also offers low freezing point and good heat transfer properties. We use RMG to generate a detailed combustion model of the complex chemical kinetics of JP-10.
 Capturing CO2 in the IGCC Process
Capturing CO2 in the IGCC Process
Integrated gasification combined cycle with carbon capture and sequestration (IGCC/CSS) is one of the most promising routes for capturing CO2 in a plant. However, the current solvent-based CO2 capture process needs to operate at low temperature, significantly decreasing the overall energy efficiency of the plant since the gas stream must be cooled, captured, then reheated. We are working on finding a solid material to capture CO2 at the preexisting process temperatures.
 HCCI Engines
HCCI Engines
Homogeneous charge compression ignition (HCCI) engines have the potential for high efficiencies and low pollutant emissions. By comparison with spark ignition (SI) engines, for example, HCCI engines can yield a 15-20% increase in fuel economy while emitting lower levels of oxides of nitrogen (NOx). Despite these advantages, however, a number of technical issues must be resolved before HCCI engines become mainstream. Many of the complications stem from the fact that HCCI engines are more sensitive to the details of the combustion chemistry than SI and diesel engines. Hence, a solid understanding of the physical and chemical processes is required to develop practical, efficient, and robust engines.

